Register Your Interest
Register your interest in the course. When we schedule dates, we will be in touch.
The price is for all three sessions
Learn how to select, manufacture and purify a Viral Vector for use in cell and gene therapies
Register your interest in the course. When we schedule dates, we will be in touch.
Learn more about the course by toggling through the tabs below. Scroll down to view the agenda, trainer info and who should attend.
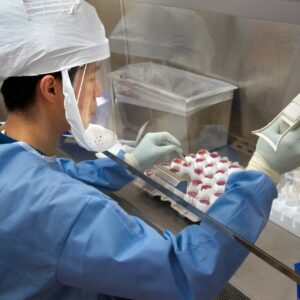
Viral Vectors play a major role in both gene and gene-modified cell therapies. When genetic materials needs to be delivered into a cell a viral vector is often used. There are significant options available to scientists depending on the application. Poor knowledge of viral vectors may lead to mishandling or inappropriate vector selection which could sabotage your therapy and compromise patient safety.
This training course will examine the underlying science of viruses and how a viral vector is manufactured. Once completed, you will have the knowledge and understanding to select the most appropriate viral vector for your therapy. You will also study in detail the purification techniques in accordance with the regulatory requirements. You will learn aspects such as: how vectors are engineered, how gene size and transfection efficiency influence vector selection and then finish off by contrasting purification requirements and techniques between vectors used for gene and gene-modified cell therapies.
With the Educo Post Learning Implementation Plan (PLIP) you will have the framework to apply and implement the knowledge acquired in the training, supporting you in your development. Learn more about how we deliver live online training.
Find out when this course is running so you can plan your training. Scroll down to view the full agenda.
If you want to train a small group, we offer a group discount between 5% and 50% depending on the course and number of attendees.
For a quote, complete our contact form by following the link. Get in touch.
We also design and deliver fully customised team training focused on your products, processes, technologies and challenges. This is ideal for specific training requirements or larger groups. Please get in touch with us for more information.
For more information, complete our contact form by following the link. Get in touch.
Learn more about the course by toggling through the tabs below (Agenda, Trainer and Who Should Attend?)
Pre-course
Action Plan
Reinforcement Session
24 September 2024 | 1:00 PM (UK)
Overview of viruses used in cell and gene therapies
180 mins | 12:00 PM UK
Understanding underlying virology and cell biology
Viral Vector manufacturing and therapeutic cell manufacturing principles
Group Exercise
This will be the start of a rolling case study. Delegates in groups will be presented with a scenario where they develop a CGT application which needs viral vectors for delivery or
engineering. Delegates will consolidate the previous two sessions and consider key steps for engineering a viral vector for their application.
180 mins | 12:00 PM UK
Choosing the most appropriate vector for your application
Choosing the most appropriate viral vector for your application
Group exercise and feedback
Groups will consider important factors and design a viral vector for their cell and
gene therapy application and feedback their thoughts to the group.
180 mins | 12:00 PM UK
Purification requirements
Purification techniques
Group exercise
In groups, delegates will consolidate the previous talks and brainstorm the purification
requirements and techniques they need to use for their particular viral vector or gene therapy
methodology rolling on from the previous group sessions.

Dr.Lars Brandén has long experience from the fields of viral and non-viral gene delivery, high throughput screening and cell based assays. Dr. Lars Brandén has a career that encompasses start-up of gene therapy companies, building and operating several high throughput cell biology centres and has worked within the life science sector as an international consultant. During his tenure in academia Dr.Brandén was mentored by professor James E. Rothman (Nobel prize winner, 2013) and recruited to several director positions with Columbia University and Yale university. Dr.Brandén has also spent a year in art school and has had international art exhibitions. Dr.Brandén is now working as Science Advisor for a global provider of automated laboratory instruments and solutions in Switzerland
This course is aimed at professionals working with or planning to work with viral vectors either as a gene therapy or as a gene modified cell therapy. Specifically, the course is aimed at those in the manufacturing of viral vectors and includes:
Module 1 of this course is free to attend and delivered online. Register to receive joining instructions for the live event, recording and presentation slides.
Discount has been applied to the price above. VAT, if applicable, will be added.
 Select options
This product has multiple variants. The options may be chosen on the product page
Select options
This product has multiple variants. The options may be chosen on the product page
 Select options
This product has multiple variants. The options may be chosen on the product page
Select options
This product has multiple variants. The options may be chosen on the product page
There is a free-to-attend training module for this course. When you register you will have full access to the live session, recording and presentation slides.
To learn more about what is covered in the module and when it is, follow the link below.

Educo Life Sciences –
This is a new course – Reviews will be added after the first delivery!